 Carbon chains form the skeletons of most organic molecules. The skeletons vary in length and may be straight, branched, or arranged in closed rings.
Variation in carbon skeletons is one important source of the molecular complexity and diversity that characterize
living matter.
Carbon chains form the skeletons of most organic molecules. The skeletons vary in length and may be straight, branched, or arranged in closed rings.
Variation in carbon skeletons is one important source of the molecular complexity and diversity that characterize
living matter. Carbon chains form the skeletons of most organic molecules. The skeletons vary in length and may be straight, branched, or arranged in closed rings.
Some carbon skeletons have double bonds, which vary in number and location. Such variation in carbon skeletons is one important source of the molecular complexity and diversity that characterize living matter. In addition, atoms of other elements can be bonded to the skeletons at available sites.
The skeletal structure of an organic compound is the series of atoms bonded together that form the essential structure of the compound. The skeleton can consist of chains, branches and/or rings of bonded atoms. Skeletal atoms other than carbon or hydrogen are called “heteroatoms”. The skeleton has hydrogen and/or various substituents bonded to its atoms. Hydrogen is the most common non–carbon atom that is bonded to carbon and, for simplicity, is not explicitly drawn.
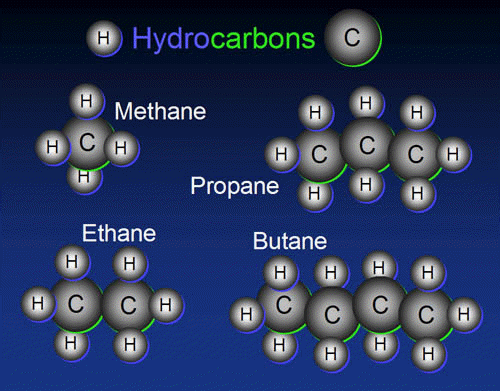 Hydrocarbons are made from hydrogen and carbon. Carbon has vacancies for four electrons in its outer shell.
So it wants to bond to four atoms. Here we see hydrocarbons with one to four carbons: methane (natural gas), ethane,
propane, and butane(lighter fluid).
Hydrocarbons are made from hydrogen and carbon. Carbon has vacancies for four electrons in its outer shell.
So it wants to bond to four atoms. Here we see hydrocarbons with one to four carbons: methane (natural gas), ethane,
propane, and butane(lighter fluid). In addition, carbon atoms are not generally labeled as such directly (i.e. with a “C”), whereas heteroatoms are always explicitly noted as such (i.e. using “N” for nitrogen, “O” for oxygen, etc.) Heteroatoms and other groups of atoms that give rise to relatively high rates of chemical reactivity, or introduce specific and interesting characteristics in the spectra of compounds are called functional groups, as they give the molecule a function. Heteroatoms and functional groups are known collectively as “substituents”, as they are considered to be a substitute for the hydrogen atom that would be present in the parent hydrocarbon of the organic compound.
The simplest organic compounds are hydrocarbons. Hydrocarbons are primitive organic compounds. They are naturally available by decay of living organism like petrol, natural gas etc. There are many examples for the hydrocarbons in our daily life. Marsh gas is made up of methane with formula CH4. Liquefied petroleum gas (LPG) is made up of Butane and isobutane. Petrol is made up of Octane. Like this other fossil fuels like diesel, crude oil are also hydrocarbons with higher chain length.
Isomers are molecules with the same molecular formula but different structures and properties. Three types of isomers are – structural isomers, geometric isomers, and enantiomers (Each of a pair of molecules that are mirror images of each other).